Becquerel
Welcome Becquerel Group
Types of Ionizing Radiation
Ionizing radiation takes a few forms: Alpha, beta, and neutron particles, and gamma and X-rays. All types are caused by unstable atoms, which have either an excess of energy or mass (or both). In order to reach a stable state, they must release that extra energy or mass in the form of radiation.
Alpha Radiation
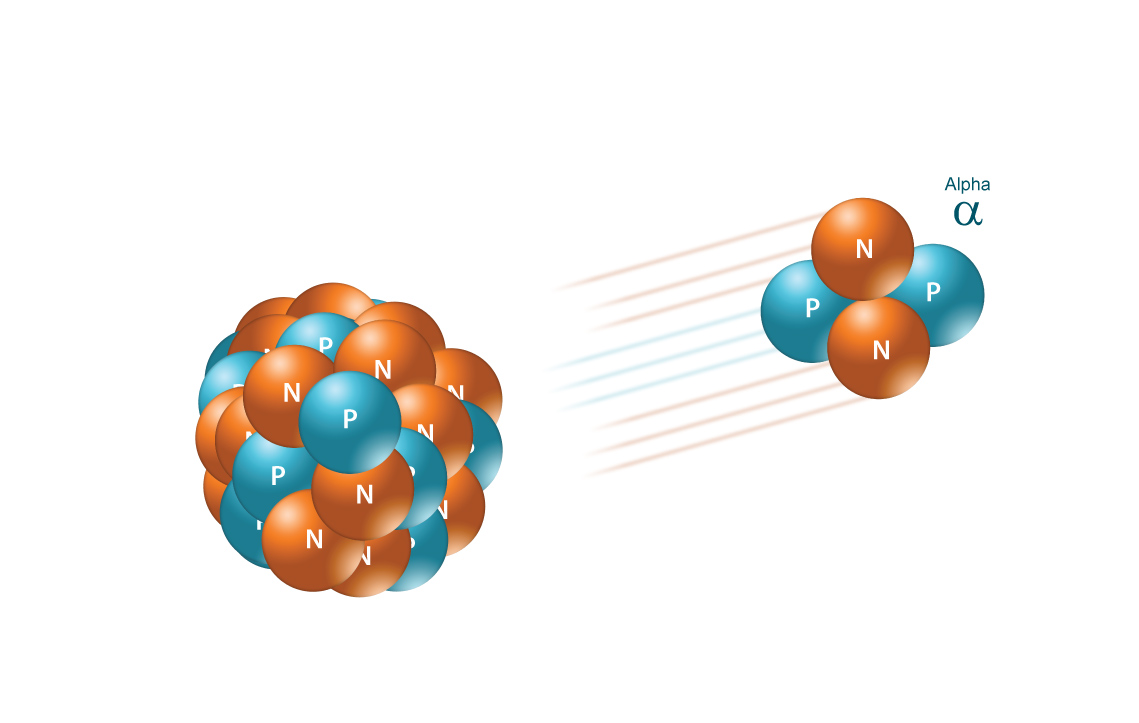
Alpha radiation occurs when an atom undergoes radioactive decay, giving off a particle (called an alpha particle) consisting of two protons and two neutrons (essentially the nucleus of a helium-4 atom), changing the originating atom to one of an element with an atomic number 2 less and atomic weight 4 less than it started with. Due to their charge and mass, alpha particles interact strongly with matter, and only travel a few centimeters in air. Alpha particles are unable to penetrate the outer layer of dead skin cells, but are capable, if an alpha emitting substance is ingested in food or air, of causing serious cell damage. Alexander Litvinenko is a famous example. He was poisoned by polonium-210, an alpha emitter, in his tea.
Beta Radiation
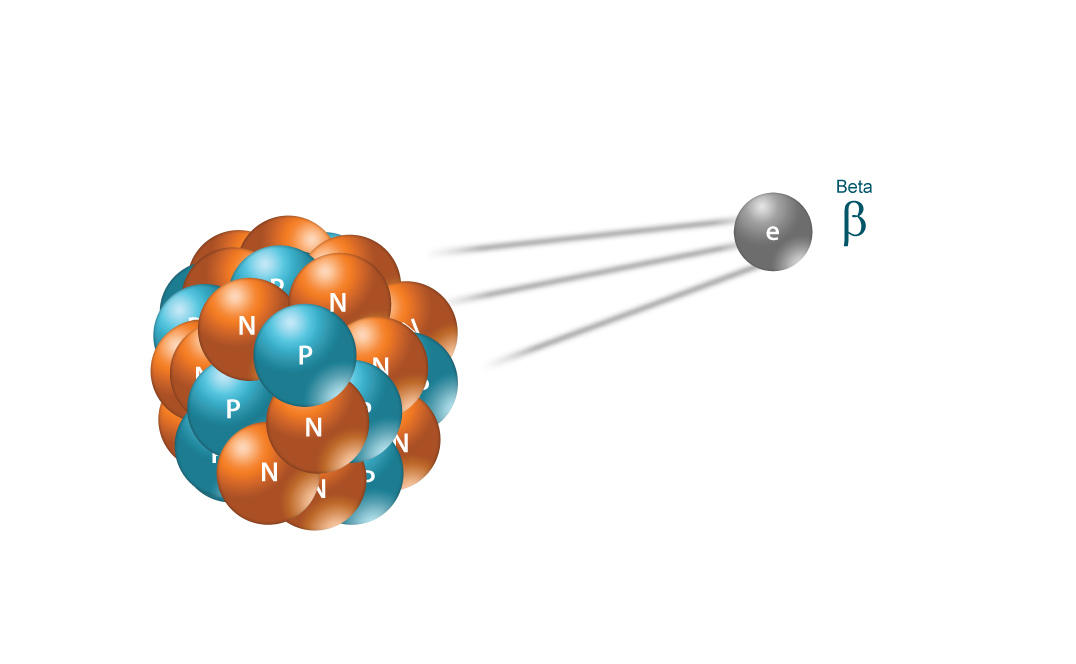
Beta radiation takes the form of fast moving electron being emitted from an atom. Due to the smaller mass, it is able to travel further in air, up to a few meters, and can be stopped by a thick piece of plastic, or even a stack of paper. It can penetrate skin a few centimeters, posing somewhat of an external health risk. However, the main threat is still primarily from internal emission from ingested material.
Gamma Radiation
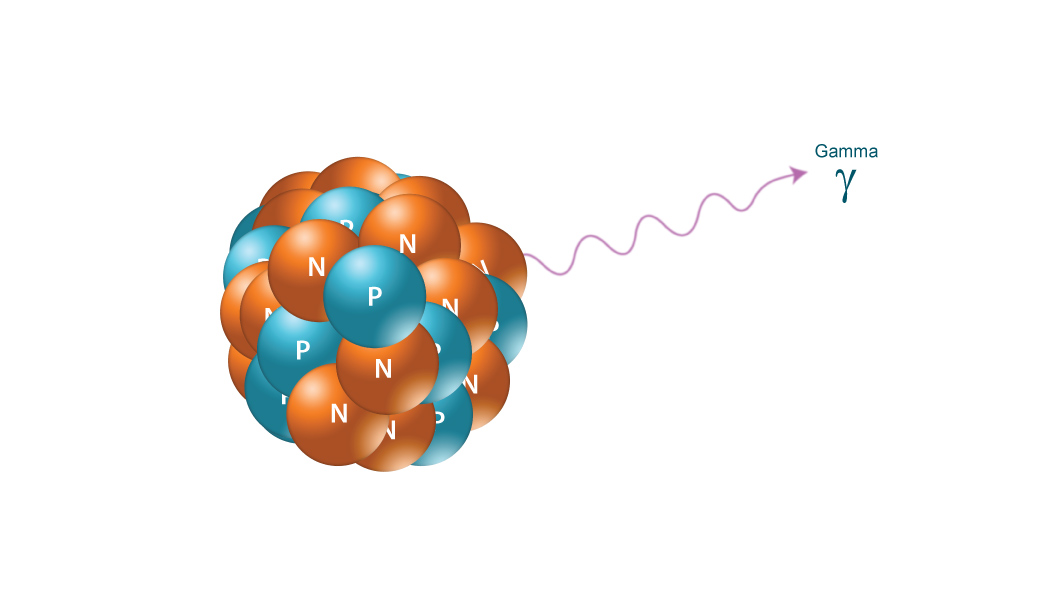
Gamma radiation, unlike alpha or beta, does not consist of any particles, instead consisting of a photon of energy being emitted from an unstable nucleus. Having no mass or charge, gamma radiation can travel much farther through air than alpha or beta, losing (on average) half its energy for every 500 feet. Gamma waves can be stopped by a thick or dense enough layer material, with high atomic number materials such as lead or depleted uranium being the most effective form of shielding.

Comments
Post a Comment